Selected Works
.png)
ChemMedChem
Rational Design and Identification of Harmine-Inspired, N-Heterocyclic DYRK1A Inhibitors Employing a Functional Genomic In Vivo Drosophila Model System
Deregulation of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) plays a significant role in developmental brain defects, early-onset neurodegeneration, neuronal cell loss, dementia, and several types of cancer. Herein, we report the discovery of three new classes of N-heterocyclic DYRK1A inhibitors based on the potent, yet toxic kinase inhibitors, harmine and harmol. An initial in vitro evaluation of the small molecule library assembled revealed that the core heterocyclic motifs benzofuranones, oxindoles, and pyrrolones, showed statistically significant DYRK1A inhibition. Further, the utilization of a low cost, high-throughput functional genomic in vivo model system to identify small molecule inhibitors that normalize DYRK1A overexpression phenotypes is described. This in vivo assay substantiated the in vitro results, and the resulting correspondence validates generated classes as architectural motifs that serve as potential DYRK1A inhibitors. Further expansion and analysis of these core compound structures will allow discovery of safe, more effective chemical inhibitors of DYRK1A to ameliorate phenotypes caused by DYRK1A overexpression.

Frontiers In Genetics
MAPPER: An open-source, high-dimensional image analysis pipeline unmasks differential regulation of Drosophila wing features
Phenomics requires quantification of large volumes of image data, necessitating high throughput image processing approaches. Existing image processing pipelines for Drosophila wings, a powerful genetic model for studying the underlying genetics for a broad range of cellular and developmental processes, are limited in speed, precision, and functional versatility. To expand on the utility of the wing as a phenotypic screening system, we developed MAPPER, an automated machine learning-based pipeline that quantifies high-dimensional phenotypic signatures, with each dimension quantifying a unique morphological feature of the Drosophila wing. MAPPER magnifies the power of Drosophila phenomics by rapidly quantifying subtle phenotypic differences in sample populations. We benchmarked MAPPER’s accuracy and precision in replicating manual measurements to demonstrate its widespread utility. The morphological features extracted using MAPPER reveal variable sexual dimorphism across Drosophila species and unique underlying sex-specific differences in morphogen signaling in male and female wings. Moreover, the length of the proximal-distal axis across the species and sexes shows a conserved scaling relationship with respect to the wing size. In sum, MAPPER is an open-source tool for rapid, high dimensional analysis of large imaging datasets. These high throughput capabilities enable rigorous and systematic identification of genotype-to-phenotype relationships in a broad range of gene screening and drug testing applications.
PLOS Computational Biology
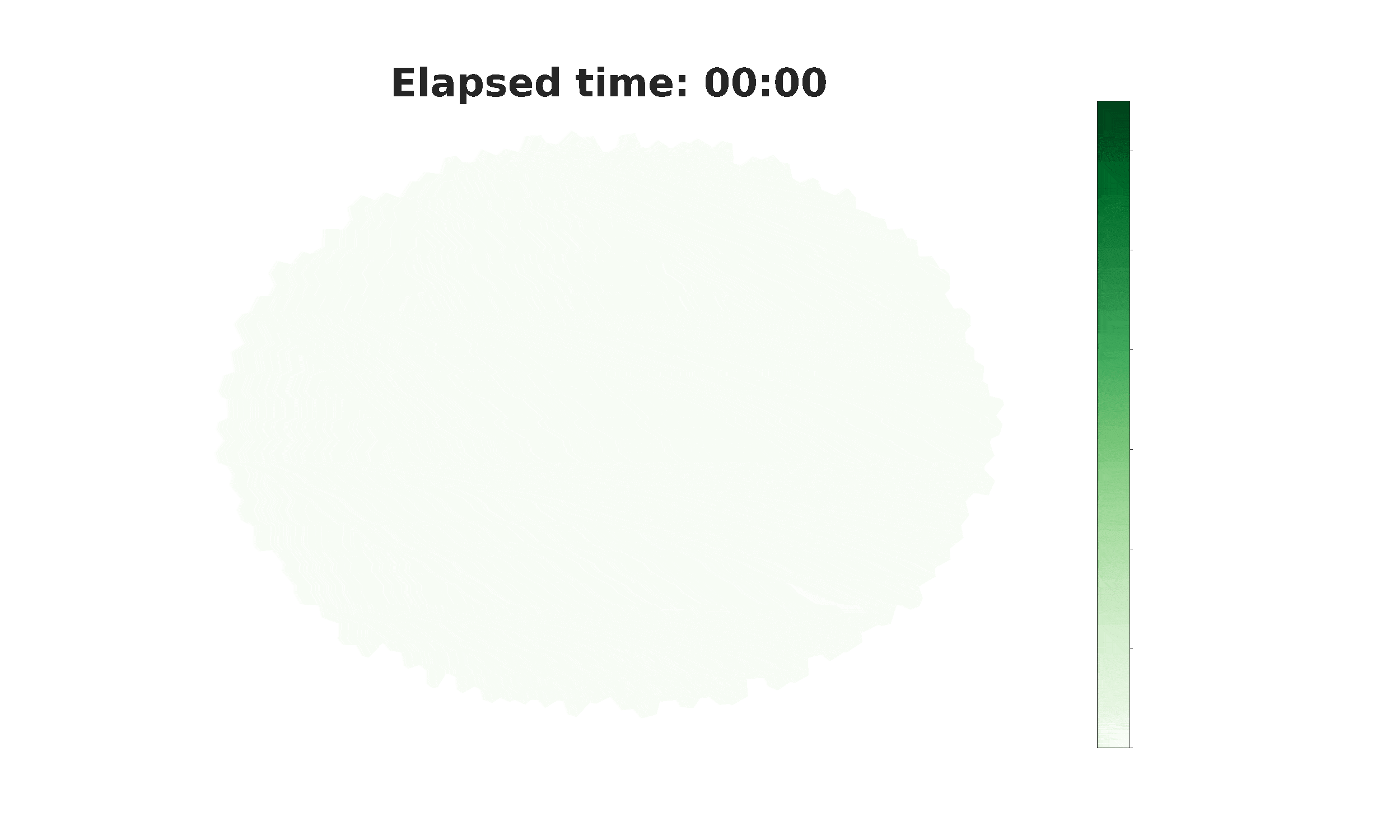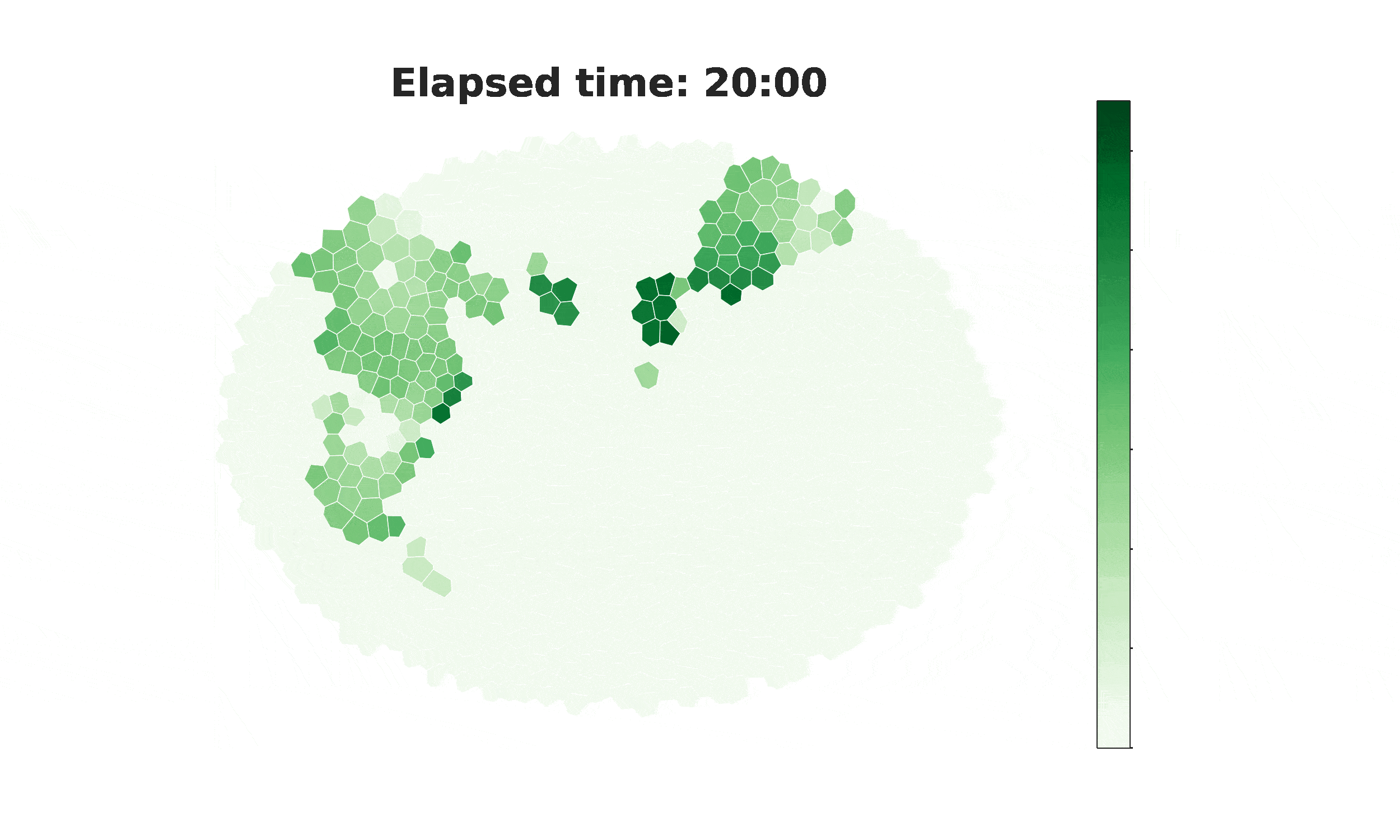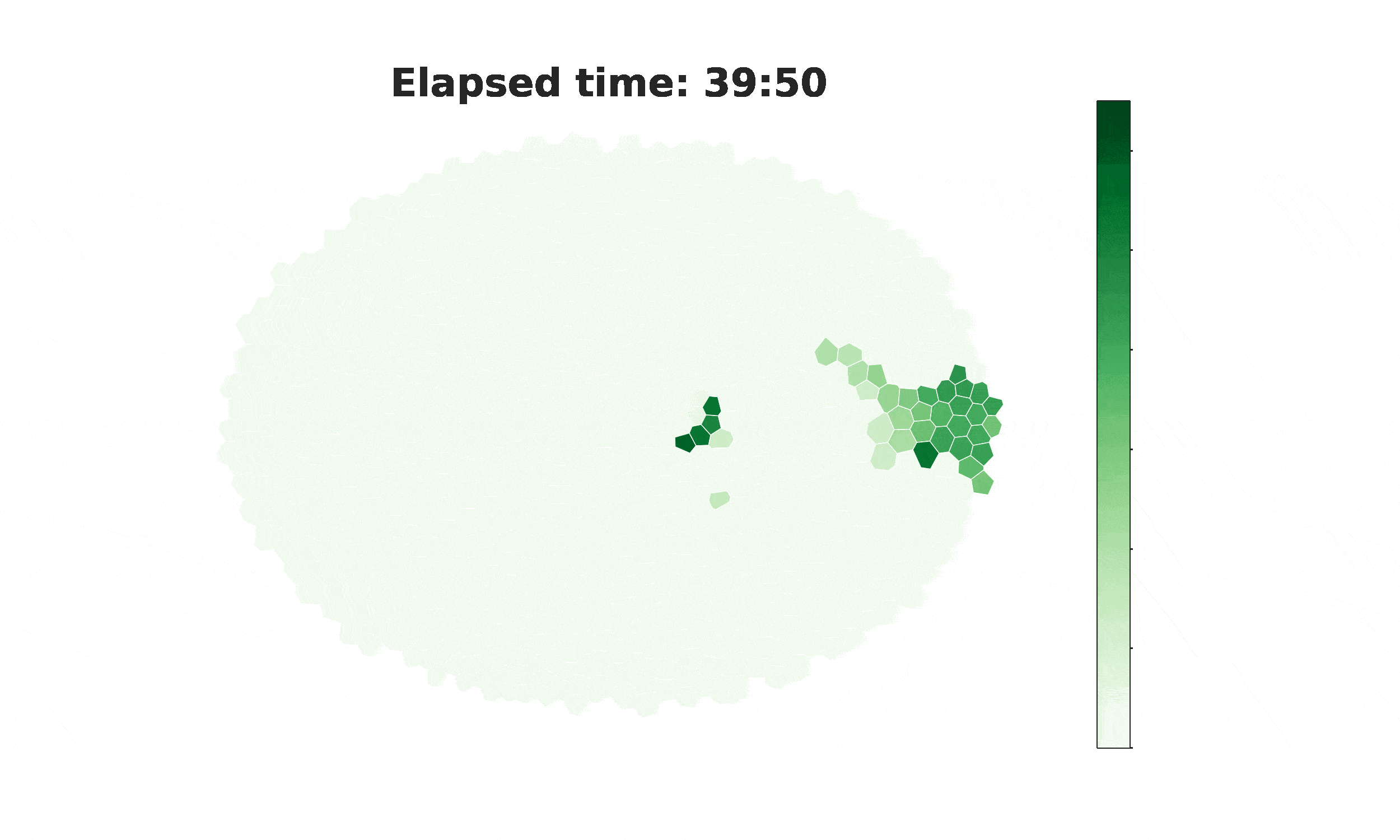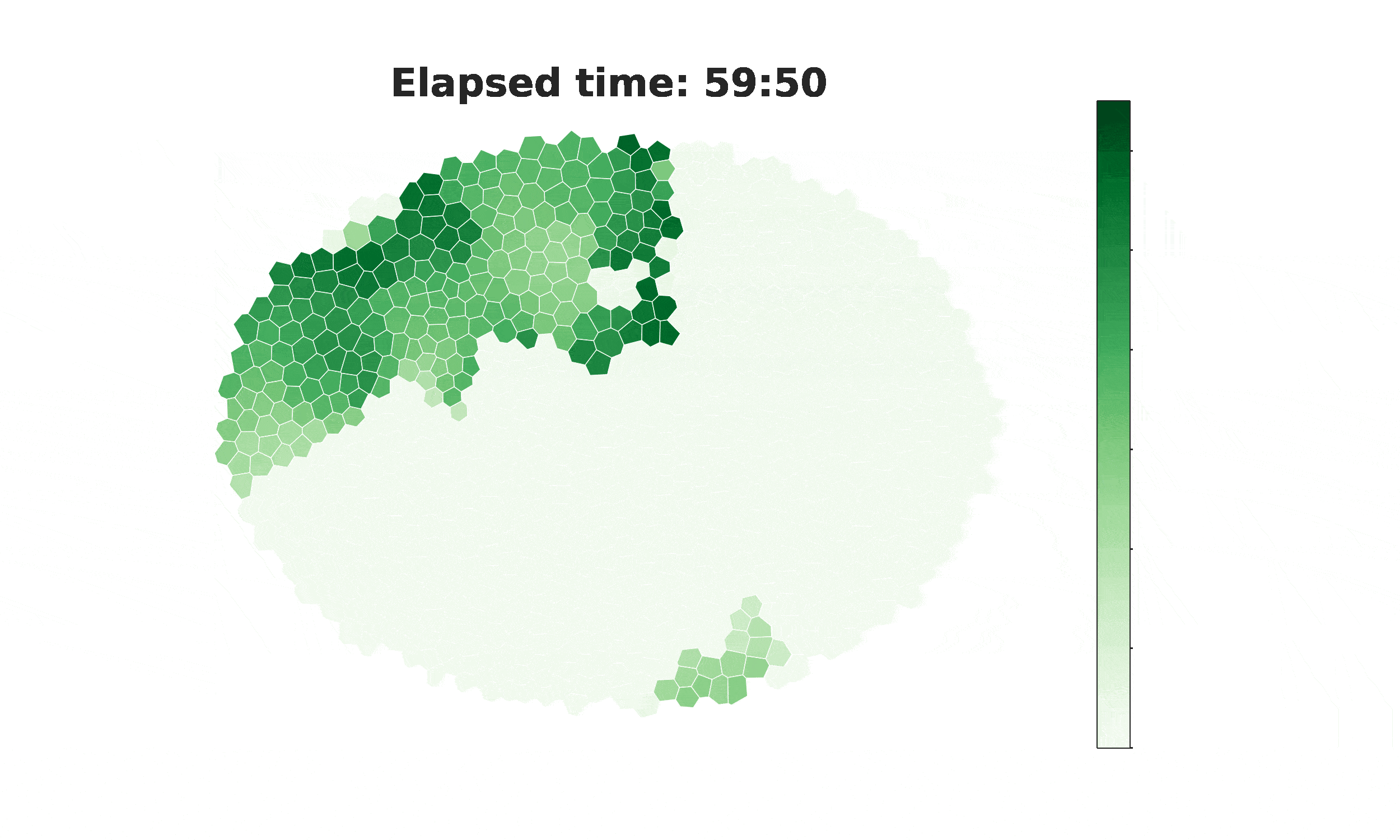
From spikes to intercellular waves: Tuning intercellular calcium signaling dynamics modulates organ size control
Information flow within and between cells depends significantly on calcium (Ca2+) signaling dynamics. However, the biophysical mechanisms that govern emergent patterns of Ca2+ signaling dynamics at the organ level remain elusive. Recent experimental studies in developing Drosophila wing imaginal discs demonstrate the emergence of four distinct patterns of Ca2+ activity: Ca2+ spikes, intercellular Ca2+ transients, tissue-level Ca2+ waves, and a global “fluttering” state. Here, we used a combination of computational modeling and experimental approaches to identify two different populations of cells within tissues that are connected by gap junction proteins. We term these two subpopulations “initiator cells,” defined by elevated levels of Phospholipase C (PLC) activity, and “standby cells,” which exhibit baseline activity. We found that the type and strength of hormonal stimulation and extent of gap junctional communication jointly determine the predominate class of Ca2+ signaling activity. Further, single-cell Ca2+ spikes are stimulated by insulin, while intercellular Ca2+ waves depend on Gαq activity. Our computational model successfully reproduces how the dynamics of Ca2+ transients varies during organ growth. Phenotypic analysis of perturbations to Gαq and insulin signaling support an integrated model of cytoplasmic Ca2+ as a dynamic reporter of overall tissue growth. Further, we show that perturbations to Ca2+ signaling tune the final size of organs. This work provides a platform to further study how organ size regulation emerges from the crosstalk between biochemical growth signals and heterogeneous cell signaling states.
GitHub: https://fjhuizar.github.io/MSELab_Calcium_Cartography_2021/
Full publication list
-
Kumar, N., †Huizar, F.J., Robinett, T., Farfan-Pira K.J., Soundarrajan, D., Unger, M., Brodskiy, P., Nahmad, M., Zartman, J.J.; Frontiers in Genetics. MAPPER: An open-source, high-dimensional image analysis pipeline unmasks differential regulation of Drosophila wing features (2022).
†Co-first authorship.
-
Huizar, F.J., Hill, H., Bacher, E., Eckert, K., Gulotty, E., Rodriguez, K., Tucker, Z., Banerjee, M., Wiest, O., Zartman, J. and Ashfeld, B.L. (2022), Rational Design and Identification of Harmine-Inspired, N-Heterocyclic DYRK1A Inhibitors Employing a Functional Genomic In Vivo Drosophila Model System. ChemMedChem. (2022).
-
Soundarrajan, D., †Huizar F.J., Paravitorghabeh, R., Robinett, T., Zartman, J. (2021), From spikes to intercellular waves: Tuning intercellular calcium signaling dynamics modulates organ size control. PLOS Computational Biology. 2021 Nov 1;17(11):e1009543.
†Co-first authorship.
-
Huizar, F.J., Soundarrajan, D., Paravitorghabeh, R, Zartman, J.J., Interplay between morphogen‐directed positional information systems and physiological signaling. Developmental Dynamics. 2020; 249: 328– 341.
-
Kumar, N., Huizar, F.J., Unger, M., Soundarrajan, D., Velagala, V., Koren, J., Zartman, J.J. (2020). Neurotransmitter Receptors as Key Physiological Regulators of Epithelial Morphogenesis. Biophysical Journal.
-
Brodskiy, P.A., Wu, Q., Soundarrajan, D.K., Huizar, F.J., Chen, J., Liang, P., Narciso, C., Levis, M., Arredondo-Walsh, N., Chen, D., Zartman, J.J. (2019). Decoding calcium signaling dynamics during Drosophila wing disc development. Biophysical Journal.
-
Brodskiy, P.A., Wu, Q., Kumar, N. Velagala, V. Snyder, K. Huizar, F.J., Tautges, S., Snyder. M, Zartman J.J. (2018). Mapping the calcium signalsome during Drosophila wing development. IFAC-PapersOnLine.
-
Wu, Q., Brodskiy, P. A., Huizar, F. J., Jangula, J. J., Narciso, C., Levis, M. K., … Zartman, J. J. (2017). In vivo relevance of intercellular calcium signaling in Drosophila wing development. bioRxiv, 187401. Pre-print.
-
Brodskiy, P. A., Wu, Q., Huizar, F. J., Soundarrajan, D. K., Narciso, C., Levis, M., … Zartman, J. J. (2017). Intercellular calcium signaling is regulated by morphogens during Drosophila wing development. bioRxiv, 104745. Pre-print.
Conference and seminar presentations
-
Huizar, F.J. et al., "Flying" over pre-clinical barriers: Machine learning approaches to characterize novel DYRK1A inhibitors as potential cancer therapeutics. Notre Dame Quantitative Biology Retreat. Poster. April 08, 2022.
-
Huizar, F.J. et al., "Flying" over pre-clinical barriers: Machine learning approaches to characterize novel DYRK1A inhibitors as potential cancer therapeutics. Cancer Research Institute Cancer Research Day. Selected graduate student presenter. Poster. March 14, 2022.
-
Huizar, F.J. et al., "Flying" over pre-clinical barriers: Development of a rapid, low-cost platform for pre-clinical evaluation of novel therapeutics in vivo. Notre Dame Bioengineering Seminar. Main speaker. Main speaker. Multidisciplinary Research Building, Notre Dame, IN. Nov 18, 2021.
-
Huizar, F.J., Hill, H., Kumar, N., Bacher, E., Ashfeld, B., Zartman, J.J., Rational Design and Identification of N-Heterocyclic DYRK1A Inhibitors as Potential Anticancer Therapeutics. Cancer Biology Training Consortium annual retreat. Poster. Graduate Hotel Nashville. Nashville, TN. Oct 25, 2021.
-
Huizar, F.J., Hill, H.M., Bacher, E.P., Eckert, K.E., Gulotty, E.M., Rodriguez, K.X., Tucker, Z.D., Wiest, O., Zartman, J., Ashfeld, B.L., Novel N-heterocyclic DYRK1A inhibitors ameliorate overgrowth of epithelial tissue. IU Simon Comprehensive Cancer Center Cancer Research Day. Presentation. Online. October 14, 2021.
-
Huizar, F.J., Using a black hole to identify novel therapeutics: SVGP regression as a statistical framework for drug discovery. Notre Dame Bioengineering Seminar. Main speaker. Online. June 22, 2021.
-
Huizar, F.J., Kumar, N., Robinett, T., Farfan-Pira K.J., Soundarrajan, D., Unger, M., Brodskiy, P., Nahmad, M., Zartman, J.J., MAPPER: A new image analysis pipeline unmasks differential regulation of Drosophila wing features. Genetics Society of America annual Drosophila research conference. Poster. Online. March 31, 2021.
-
Huizar, F.J., Hill, H., Kumar, N., Bacher, E., Ashfeld, B., Zartman, J.J., In vivo drug discovery “on-the-fly”: Towards high-throughput assessment of novel therapeutics in humanized Drosophila melanogaster. Harper Cancer Research Institute Cancer Research Day. Selected graduate student presenter. Online. March 29, 2021.
-
Huizar, F.J., Hill, H., Kumar, N., Bacher, E., Ashfeld, B., Zartman, J.J., In vivo drug discovery “on-the-fly”: Towards high-throughput assessment of novel therapeutics in humanized Drosophila melanogaster. Cancer Biology Training Consortium annual retreat. Poster. Online. Oct 27, 2020.
-
Huizar, F.J., Hill, H., Kumar, N., Bacher, E., Ashfeld, B., Zartman, J.J., In vivo drug discovery “on-the-fly”: Towards high-throughput assessment of novel therapeutics in humanized Drosophila melanogaster. Notre Dame Bioengineering Seminar. Main speaker. Multidisciplinary Research Building, Notre Dame, IN. Sep 29, 2020.
-
Maini, P., Huizar F.J., Oliveri, H., Baraban, M., Kumar, N., Parel, K., Telele. C., Modeling shape & size in biological multicellular tissues. Workshop Presentation. Lorentz Center modeling biological development workshop. Online. Aug 28, 2020.
-
Huizar, F.J., Bacher, E., Kumar, N., Gleason, B., Koren, J., Ashfeld, B., Zartman, J.J., Regulation of DYRK1A activity through an integrated inhibitor scaffold design and phenotypic analysis approach. Poster. 7th Annual AD&T Symposium: Extreme Diagnostics, Notre Dame, IN. Mar 6, 2020.
-
Huizar, F.J., Wu, Q., Tautges, S., Eckert, K., Bacher, E., Ashfeld, B., Zartman, J.J., Toward a high-throughput in vivo screening pipeline for testing new therapeutics using Drosophila melanogaster as a disease-model. Eli Lilly and Company HCRI Notre Dame Alumni Panel. Poster. Eli Lilly and Company Headquarters, Indianapolis, IN. May 17, 2019.
-
Huizar, F.J., Drosophila melanogaster as a high-throughput model for drug screening and decoding G-protein coupled receptor interactions. Notre Dame Bioengineering Seminar. Main speaker. Multidisciplinary Research Building, Notre Dame, IN. Apr 12, 2019.
-
Huizar, F.J., Bacher, E., Wu, Q., Tautges, S., Kumar, N., Ashfeld, B., Zartman, J.J., A high-throughput in vivo screening pipeline for testing novel DYRK1A inhibitors against triple negative breast cancer using Drosophila melanogaster as a disease model. 8th Annual HCRI Cancer Research Day. Selected Presenter. Harper Cancer Research Institute, South Bend, IN. Apr. 8, 2019.
-
Huizar, F.J., Wu, Q., Tautges, S., Eckert, K., Bacher, E., Ashfeld, B., Zartman, J.J., Toward a high-throughput in vivo screening pipeline for testing new therapeutics using Drosophila melanogaster as a disease-model. 8th Annual HCRI Cancer Research Day. Poster. Harper Cancer Research Institute, South Bend, IN. Apr 8, 2019.
-
Huizar, F.J., Mechanisms of serotonin (5-HT) signaling in development. Harper Cancer Research Institute Seminar. Main speaker. Harper Cancer Research Institute, South Bend, IN. Feb 4, 2019.
-
Huizar, F.J., Wu, Q., Brodskiy, P.A., Zartman, J.J., Regulation and relevance of intercellular calcium signaling. Poster. Notre Dame College of Science Joint Annual Meeting, Notre Dame, IN. May 4, 2018.
-
Huizar, F.J., Levis, M., Zartman J.J., In vivo analysis of spontaneous intercellular Ca2+ waves in Drosophila wing discs. Poster. 5th Midwest Quantitative Biology Symposium, Notre Dame, IN. Apr 8, 2017.






